A recent article discusses the surgeon's responsibility for the use of new technologies:
The bullet points are
➢ The introduction of new devices, biologics, and combination products to the orthopaedic marketplace is increasing rapidly.
➢ The majority of these new technologies obtain clearance to market by demonstrating substantial equivalence to a predicate (previously approved device) according to the U.S. Food and Drug Administration (FDA) 510(k) process.
➢ Surgeons play a critical role in the introduction of new technologies to patients and must take a leadership role in promoting safe, efficacious, appropriate, and cost-effective care, especially for operative procedures.
➢ Surgeons should monitor and document their patients’ clinical outcomes and adverse events when using new technology, to ensure that the new technology is performing as desired.
This fits right in with a prior post:
Analysis of FDA-Approved Orthopaedic Devices and Their Recalls.
These authors note that there are two paths by which medical devices, such as shoulder implants, can obtain approval for use by the U.S. Food and Drug Administration (FDA). The more stringent Premarket Approval (PMA) review requires clinical trials, and the Premarket Notification 510(k) process generally exempts devices from clinical trials if they prove to be "substantially equivalent" to existing devices.
They hypothesized that because 510(k) approval was less stringent, it would be more commonly used on one hand and devices approved by this mechanism would be more likely to be recalled.
They searched for the following: PMA and 510(k) clearances for orthopaedics and non-orthopaedic specialties from 1992 to 2012. They also searched for all device recall events from 2002 to 2012. For the top-twenty recall companies, they calculated the odds ratio that compares the likelihood of recall for 510(k)-approved devices with that for PMA-approved devices.
While non-orthopaedic devices are increasingly approved by PMA:
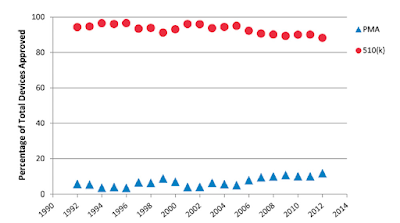
Orthopaedic devices continue to be approved principally by 510(k):
The type of approval process is strongly related to the frequency of recall:from 2002 to 2012, the percentage of recalled devices was 17.8% for 510(k)-cleared devices and 1.6% for PMA-approved devices.
They conclude that 510(k)-cleared devices were 11.5 times more likely to be recalled than PMA-approved devices; therefore is concerning that most orthopaedic devices are cleared through the 510(k) process with limited clinical trials data.
Comment: These data suggest that the 510(k) process, being easier and less expensive, is being used for devices that are not, in fact, "substantially equivalent to existing devices. " If they were "substantially equivalent", the recall rate discrepancy would not be what it is. It may be time to re-look at what it takes to qualify for 510(k) approval.
When we see data, such as that shown below from the AOA registry, it makes us wonder how "new" implants come to market, and which ones were claimed to be "substantially equivalent".
Comment: These data suggest that the 510(k) process, being easier and less expensive, is being used for devices that are not, in fact, "substantially equivalent to existing devices. " If they were "substantially equivalent", the recall rate discrepancy would not be what it is. It may be time to re-look at what it takes to qualify for 510(k) approval.
When we see data, such as that shown below from the AOA registry, it makes us wonder how "new" implants come to market, and which ones were claimed to be "substantially equivalent".
===
Use the "Search" box to the right to find other topics of interest to you.
You may be interested in some of our most visited web pages including:shoulder arthritis, total shoulder, ream and run, reverse total shoulder, CTA arthroplasty, and rotator cuff surgery as well as the 'ream and run essentials'
You may be interested in some of our most visited web pages including:shoulder arthritis, total shoulder, ream and run, reverse total shoulder, CTA arthroplasty, and rotator cuff surgery as well as the 'ream and run essentials'