These authors report a metallurgic analysis of the fractured humeral tray of a reverse total shoulder humeral component using Scanning Electron Microscopy (SEM) and Electron Dispersion Spectroscopy (EDS). The atraumatic failure occurred four years ofter the arthroplasty by dissociation of the taper from the humeral tray at the weld, leaving the Morse taper embedded in the humeral stem while the tray floated freely in the patient’s shoulder.
SEM further confirmed the jagged edges noted grossly at the weld fracture site, both suggesting failure due to torsional forces.
EDS detected elevated levels of carbon and oxygen at the fracture site on the taper. In order to determine the origin of the high levels of C and O, it was considered that in titanium alloys, C and O are used as stabilizers that help raise the temperature at which titanium can be cast. Since the presence of stabilizers reduces ductility and fatigue strength, all interstitial elements are removed after casting. Considering this, the presence of C and O suggests that not all of the interstitials were removed during the manufacturing process, resulting in decreased fatigue strength. In other words residual C and O in the taper lowered the metal implant’s integrity, leading to torsional cracking at the weld junction of the humeral tray and the taper. The elevated levels of C and O measured at fracture sites on both the tray and the taper suggest poor quality filler metal or failure to remove all interstitial elements after casting. The authors suggest that the system was undersized, considering the actions the shoulder is expected to be able to carry out. The short length of the Morse taper, thinness of the humeral tray, weaker metal system, and small surface area of the weld site between the taper and tray all posed probable causes of failure. The result was decreased fatigue strength and overall toughness, leading to mechanical failure.
This design has been recalled after a number of similar failures.
Comment: New shoulder implants are being introduced to the marketplace each year. Many of these have a modular design, which necessitates a junction between different component elements. Each of these junctions represents a site for potential corrosion and fatigue failure.
While it is tempting for surgeons to assume that "FDA approval" means that the device is safe, we must recognize that this approval usually does not include a careful trial of these implants in human subjects before they are released for general use. As a result, delayed failures - as represented by this report - may not become evident until after the implant has been out in general use for years. When design-related failures occur, years may pass before enough data are collected for a product recall.
Thus the surgeon needs to become a careful consumer, knowledgeable about metallurgy, fatigue and corrosion.
A recent article discusses the surgeon's responsibility for the use of new technologies:
The bullet points are
➢ The introduction of new devices, biologics, and combination products to the orthopaedic marketplace is increasing rapidly.
➢ The majority of these new technologies obtain clearance to market by demonstrating substantial equivalence to a predicate (previously approved device) according to the U.S. Food and Drug Administration (FDA) 510(k) process.
➢ Surgeons play a critical role in the introduction of new technologies to patients and must take a leadership role in promoting safe, efficacious, appropriate, and cost-effective care, especially for operative procedures.
➢ Surgeons should monitor and document their patients’ clinical outcomes and adverse events when using new technology, to ensure that the new technology is performing as desired.
This fits right in with a prior post:
Analysis of FDA-Approved Orthopaedic Devices and Their Recalls.
These authors note that there are two paths by which medical devices, such as shoulder implants, can obtain approval for use by the U.S. Food and Drug Administration (FDA). The more stringent Premarket Approval (PMA) review requires clinical trials, and the Premarket Notification 510(k) process generally exempts devices from clinical trials if they prove to be "substantially equivalent" to existing devices.
They hypothesized that because 510(k) approval was less stringent, it would be more commonly used on one hand and devices approved by this mechanism would be more likely to be recalled.
They searched for the following: PMA and 510(k) clearances for orthopaedics and non-orthopaedic specialties from 1992 to 2012. They also searched for all device recall events from 2002 to 2012. For the top-twenty recall companies, they calculated the odds ratio that compares the likelihood of recall for 510(k)-approved devices with that for PMA-approved devices.
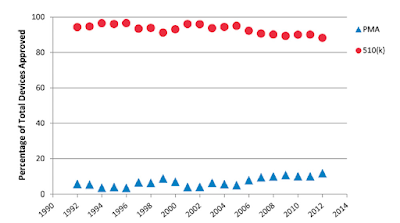
While non-orthopaedic devices are increasingly approved by PMA:
Orthopaedic devices continue to be approved principally by 510(k):
The type of approval process is strongly related to the frequency of recall:from 2002 to 2012, the percentage of recalled devices was 17.8% for 510(k)-cleared devices and 1.6% for PMA-approved devices.
They conclude that 510(k)-cleared devices were 11.5 times more likely to be recalled than PMA-approved devices; therefore is concerning that most orthopaedic devices are cleared through the 510(k) process with limited clinical trials data.
These data suggest that the 510(k) process, being easier and less expensive, is being used for devices that are not, in fact, "substantially equivalent to existing devices. " If they were "substantially equivalent", the recall rate discrepancy would not be what it is. It may be time to re-look at what it takes to qualify for 510(k) approval.
When we see data, such as that shown below from the AOA registry, it makes us wonder how "new" implants come to market, and which ones were claimed to be "substantially equivalent".
These data suggest that the 510(k) process, being easier and less expensive, is being used for devices that are not, in fact, "substantially equivalent to existing devices. " If they were "substantially equivalent", the recall rate discrepancy would not be what it is. It may be time to re-look at what it takes to qualify for 510(k) approval.
When we see data, such as that shown below from the AOA registry, it makes us wonder how "new" implants come to market, and which ones were claimed to be "substantially equivalent".
The question also arises, "with the dramatic increase in the number of new implants that are being introduced are patients getting better results?" See this recent post.
Is there evidence that the outcomes of primary anatomic and reverse shoulder arthroplasty are getting better?
These authors noted that the number of shoulder arthroplasty implants and related devices approved by the FDA are improving exponentially with time.
These new devices increase the cost of shoulder arthroplasty surgery because of their associated development, FDA approval and marketing costs as well as the learning curves and uncertainty of outcomes in comparison to devices that have been in use for longer periods of time.
The authors sought to use published evidence from studies published from 1990 to 2015 to answer the question, "are the patient-reported outcomes and re-operation rates better in reports of more recently performed anatomic (TSA) and reverse (RSA) total shoulder arthroplasties?" The difficulty in answering such a question lies in the fact that the study methods and patient cohorts differ among different reports, confounding attempts to compare the results.
Inclusion criteria for this investigation were met by 42 TSA studies with a mean (± SD) of 116±159 (range, 20–705) patients per study with an average follow-up of 5±3 years, 42±21% males with average age 66±5 years. Inclusion criteria were met by 37 RSA studies with 56±32 (20–174) patients per study with an average follow-up of 4±5 years, 34±15% males with average age 72±5 years.
Inclusion criteria for this investigation were met by 42 TSA studies with a mean (± SD) of 116±159 (range, 20–705) patients per study with an average follow-up of 5±3 years, 42±21% males with average age 66±5 years. Inclusion criteria were met by 37 RSA studies with 56±32 (20–174) patients per study with an average follow-up of 4±5 years, 34±15% males with average age 72±5 years.
In order to compare studies that used different outcome scales (ASES, SST, Constant, Dash, SANE, etc), the authors normalized each scale to a 0 (worst) to 100 (best) outcome score. They considered the outcome in terms of the final post-operative score as well as the percent of maximal possible improvement (%MPI). As shown in the figure below, the particular outcome scale used in the different publications had a major effect on the normalized outcome score for shoulder arthroplasty. For TSA, the mean normalized post-operative scores were ≥80% for reports using the WOOS, UCLA, Penn, and SANE scales and <65% using the DASH or Constant scales. For RSA, the mean normalized postoperative scores were ≥70% for the Oxford, ASES and VAS Pain scales and ≤60% for the SANE and UCLA scales. For both TSA and RSA the Simple Shoulder Test was at the median.
The diagnosis for which the arthroplasty was performed presented another confounder in the comparison among studies. For reports of TSA, osteoarthritis (OA) was most common diagnosis (73% for an average study), but the percentage of patients with this diagnosis varied greatly from study to study (0–100%). For reports of RSA, the most common diagnosis was cuff tear arthropathy (CTA) (48% for an average study) but, again, the percentage of patients with this diagnosis varied greatly from study to study (0-100%). For both types of arthroplasty, the diagnosis had a significant effect on the outcome as shown in the figure below. For TSA the results were worse in those studies of rheumatoid arthritis (RA) and cuff tear arthropathy (CTA). Clinical outcomes for RSA were worse in studies of post-traumatic arthritis (PTA).
Over the two decades of this study, there were marginally significantly better clinical outcomes in reports of more recently performed TSAs (p = 0.048). The plots below show the mean outcome score adjusted for outcome scale and diagnosis by median year of surgery.
For RSA the trend showed no significant improvement.
Neither the revision rate for TSA (Coefficient -0.48, 95% CI from -1.35 to 0.39, p= 0.3) or the revision rate for RSA (Coefficient -0.84, 95% CI (from -2.87 to 1.19, p= 0.4) were significantly lower in studies reporting more recently performed procedures.
It appears that better evidence will be necessary to demonstrate that newer implants and techniques are yielding improved clinical outcomes for patients with glenohumeral arthritis.
It appears that better evidence will be necessary to demonstrate that newer implants and techniques are yielding improved clinical outcomes for patients with glenohumeral arthritis.
The authors suggest that future studies reporting the results of shoulder arthroplasty should include an appendix containing a set of basic data elements for each patient so that meaningful comparisons can be facilitated. Such a data set should include for each patient the age, sex, diagnosis, the scale used to document the presurgical and postoperative patient self-assessed shoulder comfort and function, and date and reason for any reoperation. While this minimal data set will not capture the full set of potential confounders— such as the degree of shoulder stiffness, the condition of the rotator cuff, radiographic pathoanatomy and the effect of surgical team volume and experience—this degree of standardization will enable a more robust comparison of the outcomes for individual patients treated over time with different therapeutic approaches, so that we can learn whether newer implants and techniques contribute added value to the patient with glenohumeral arthritis.
Supporting progress in shoulder surgery
Consultation for those who live a distance away from Seattle.
Click here to see the new Shoulder Arthritis Book
Click here to see the new Rotator Cuff Book
You may be interested in some of our most visited web pages including:shoulder arthritis, total shoulder, ream and run, reverse total shoulder, CTA arthroplasty, and rotator cuff surgery as well as the 'ream and run essentials'
See from which cities our patients come.
See the countries from which our readers come on this post.
Consultation for those who live a distance away from Seattle.
Click here to see the new Shoulder Arthritis Book
Click here to see the new Rotator Cuff Book
You may be interested in some of our most visited web pages including:shoulder arthritis, total shoulder, ream and run, reverse total shoulder, CTA arthroplasty, and rotator cuff surgery as well as the 'ream and run essentials'
See from which cities our patients come.
See the countries from which our readers come on this post.
Use the "Search" box to the right to find other topics of interest to you.