Role of rifampin against Propionibacterium acnes biofilm in vitro and in an experimental foreign-body infection model.
These authors began with a nice summary: "propionibacterium acnes is a facultative anaerobic Gram-positive branching rod physiologically residing in sebaceous glands of the skin. It is the major agent of inflammatory acne. In addition, in 2 to 14% of cases, it is identified as the cause of various implant-associated infections, including prosthetic-joint infections, particularly shoulder prosthesis; spine implant surgery; breast implant surgery; electrophysiological cardiac devices; and neurosurgery involving ventricular drains and ventriculoperitoneal shunts. The role of P. acnes in foreign-body infections is probably underestimated due to technical reasons. Detection of anaerobes requires rapid transport to the microbiology laboratory or special transport media and needs incubation for up to 14 days due to slow growth. Late growth and/or growth in enrichment media only is often misinterpreted as contamination. Furthermore, although P. acnes is usually introduced during surgery, clinical symptoms of lowgrade infections often manifest only months to years after implantation.
Therefore, the association between implant surgery and infection is not always obvious. Recent studies showed that P. acnes forms biofilm on a wide range of materials. However, little is known about the mechanisms involved in biofilm formation at the cellular and molecular levels. P. acnes is uniformly resistant to metronidazole but susceptible to several other antimicrobials, including penicillin G, ceftriaxone, vancomycin, and clindamycin. However, the antimicrobial susceptibility is significantly reduced in biofilms, causing chronic and persistent infections that are difficult to cure
without removal of the device. In addition, P. acnes can escape the immune response by resisting phagocytosis and surviving inside macrophages"
They investigated the activity of rifampin, alone and in combination, against planktonic and biofilm P. acnes in vitro and in a foreign-body infection model (polytetrafluorethylene (Teflon) cages with 130 regularly spaced perforations 1 mm in diameter subcutaneously implanted in the flanks of guinea pigs).
To determine the activity against planktonic P. acnes, cage fluid was aspirated before the start of treatment, during treatment (before administration of the last dose), and 5 days after completion of treatment.
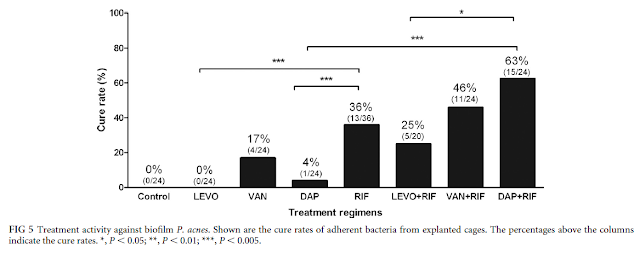
To determine the activity against biofilm P. acnes, animals were sacrificed 5 days after completion of treatment, and the cages were explanted under aseptic conditions and incubated for 10 days in BHI. The treatment efficacy against adherent bacteria was expressed as the cure rate (as a percentage) defined as the number of cages without P. acnes growth divided by the total number of cages in the individual treatment group.
When a high infection inoculum was injected into the tissue cage fluid of guinea pigs, P. acnes persisted on implanted cages for 50 days, despite spontaneous clearance of planktonic P. acnes from aspirated cage fluid.
They found that MIC and the minimal bactericidal concentration (MBC) were 0.007 and 4 μg/ml for rifampin, 1 and 4 μg/ml for daptomycin, 1 and 8 μg/ml for vancomycin, 1 and 2 μg/ml for levofloxacin, 0.03 and 16 μg/ml for penicillin G, 0.125 and 512 μg/ml for clindamycin, and 0.25 and 32 μg/ml for ceftriaxone.
The P. acnes minimal biofilm eradication concentration (MBEC) was 16 μg/ml for rifampin; 32 μg/ml for penicillin G; 64 μg/ml for daptomycin and ceftriaxone; and ≥128 μg/ml for levofloxacin, vancomycin, and clindamycin.
In the animal model, implants were infected by injection of 10⁹ CFU P. acnes in cages. Antimicrobial activity on P. acnes was investigated in the cage fluid (planktonic form) and on explanted cages (biofilm form).
When a high infection inoculum was injected into the tissue cage fluid of guinea pigs, P. acnes persisted on implanted cages for 50 days, despite spontaneous clearance of planktonic P. acnes from aspirated cage fluid.
This finding highlights the great ability of P. acnes to adhere to the implant surface and its change from the planktonic to the biofilm phenotype.
Based on their vitro biofilm studies, the combination of rifampin and penicillin G or ceftriaxone
may represent alternative options, but they were not able to investigate this in their animal model, since guinea pigs do not tolerate Beta-lactams and clindamycin (because of gastrointestinal disturbance).
The MIC values of all tested drugs for this strain were low. In contrast, the MBCs of commonly used antimicrobials, such as penicillin G (16 g/ml), ceftriaxone (32 g/ml), and clindamycin (512 g/ml), were high for P. acnes infections. Interestingly, rifampin, daptomycin, and levofloxacin demonstrated low MBCs (4 g/ml), suggesting superior killing of planktonic P. acnes.
For the biofilms, the cure rates were 4% for daptomycin, 17% for vancomycin, 0% for levofloxacin, and 36% for rifampin. Rifampin cured 63% of the infected cages in combination with daptomycin, 46% with vancomycin, and 25% with levofloxacin.
While all tested antimicrobials showed good activity against planktonic P. acnes, for eradication of biofilms, rifampin was needed. In combination with rifampin, daptomycin showed higher cure rates than with vancomycin in this foreign-body infection model.
may represent alternative options, but they were not able to investigate this in their animal model, since guinea pigs do not tolerate Beta-lactams and clindamycin (because of gastrointestinal disturbance).
Comment: This is a very helpful study. One of the most interesting findings is the fluid around a Propionibacterium culture positive biofilm becomes sterile over time. This explains why periprosthetic joint fluid that is culture negative for Propionibacterium does not rule out the presence of Propionibacterium on the implant.
A second interesting finding is that the antibiotic levels necessary to affect the Propionibacterium in biofilms is much higher than that for planktonic bacteria.
Thirdly, Rifampin - a drug that may be challenging for patients to take - has a relatively strong effect on biofilm bacteria.
Fourthly, even with Rifampin, the 'cure rate' is not great - removal of the implant is the only way to resolve the infection.
===
Click here to see the new Rotator Cuff Book
Information about shoulder exercises can be found at this link.
Information about shoulder exercises can be found at this link.
Use the "Search" box to the right to find other topics of interest to you.
You may be interested in some of our most visited web pages including:shoulder arthritis, total shoulder, ream and run, reverse total shoulder, CTA arthroplasty, and rotator cuff surgery as well as the 'ream and run essentials'
You may be interested in some of our most visited web pages including:shoulder arthritis, total shoulder, ream and run, reverse total shoulder, CTA arthroplasty, and rotator cuff surgery as well as the 'ream and run essentials'