Backside Polyethylene Wear in Reverse Shoulder Arthroplasty
These authors point out that the particles from polyethylene wear may result in osteolysis and component loosening in reverse shoulder arthroplasty (RSA) - this wear may occur on either the articular side of the polyethylene (PE) or on the backside of the PE liner.
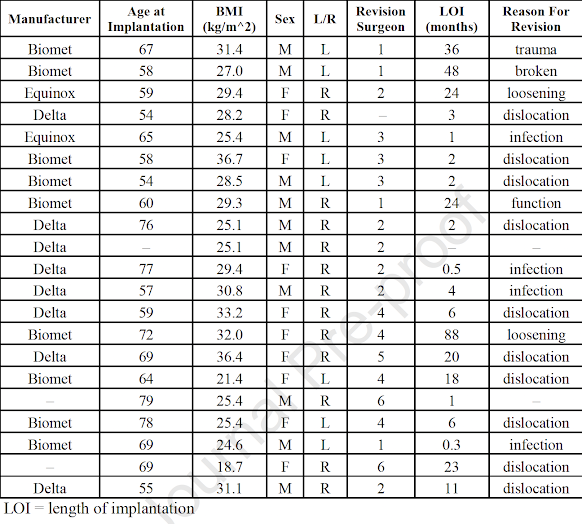
They sought to characterize the damage to the backside and articular surfaces in 21 explanted RSA components.
The mean time between implantation and extraction was 16 months (10 days–88 months). Diagnoses at the time of revision included dislocation (10), infection (4), mechanical failure (3), loosening (2), and unknown (2).
Damage was noted on the articular surfaces of all 21 liners and on the backside surface of 20 liners. The total damage in all the quadrants was higher on the articular surface than on the backside of the component. The articular side exhibited greater scratching, abrasion, and surface deformation than the backside.The inferior anatomical aspect showed the greatest amount of wear.
It is of interest that dislocation was the most common reason for RSA revision in this series, followed by infection. The length of implantation ranged from 0.3 to 88 months.
These authors used a coordinate measurement machine (CMM) and custom computer algorithms to quantify the volumetric wear of polyethylene humeral bearings. This technique was validated using two
never-implanted polyethylene humeral liners with a controlled amount of wear in clinically relevant locations. The technique was determined to be accurate to within 10% of the known value and within 5mm^3 of the gravimetrically determined values.
Following validation, ten retrieved polyethylene humeral liners were analyzed to determine a baseline for future clinical tests. Four of the ten polyethylene humeral liners showed visible and measureable wear volumes ranging from 40 to 90mm^3 total with a maximum wear rate as high as 470mm^3/year in one short duration and significantly damaged humeral liner.
They found dosages of shed polyethylene were well within the osteolytic thresholds that have been suggested, indicating that osteolysis may be a clinical concern in the shoulder.
Follow on twitter: https://twitter.com/shoulderarth
Follow on facebook: https://www.facebook.com/frederick.matsen
Follow on LinkedIn: https://www.linkedin.com/in/rick-matsen-88b1a8133/
How you can support research in shoulder surgery Click on this link.
Here are some videos that are of shoulder interest