These authors observe that perioperative hyperglycemia can have a detrimental effect on postoperative outcomes in patients without diabetes, but that it has not been established if the treatment of patients without diabetes is safe and effective.
They hypothesized that sliding-scale insulin for severe postoperative hyperglycemia (glucose ≥180 mg/dL) could lower mean postoperative glucose levels and minimize short-term complications in patients without diabetes undergoing major joint replacement.
Patients had a fasting glucose measurement taken on the morning of the surgical procedure. Point-of-care finger-stick glucose samples were taken in the post-anesthesia care unit (PACU), pre-dinner, at bedtime, and in the morning after the surgical procedure. Sliding scale insulin (lispro) was used to treat severe hyperglycemia in patients without diabetes.
They compared a prospective study group of 1,398 consecutive patients, with and without diabetes, undergoing joint replacement who were monitored and treated for hyperglycemia to a group of 886 historical, less frequently monitored control patients.
Multivariable linear regression revealed factors that contributed to elevated mean glucose after arthroplasty in patients without diabetes: preoperative fasting glucose (p < 0.001), perioperative steroid use (p < 0.001), general anesthesia (p < 0.001), procedure duration (p = 0.003), and transfusion (p = 0.008). The most important variable predicting elevated postoperative glucose for both patients with diabetes and patients without diabetes was preoperative fasting glucose.
Of 968 patients without diabetes, 203 developed severe hyperglycemia. The recommended insulin coverage was given to 129 of these patients, and 74 patients did not receive it for various clinical
reasons. They found that in comparison to the historical controls, sliding-scale insulin for severe postoperative hyperglycemia (glucose ≥180 mg/dL) lowered the mean glucose for patients without diabetes.
No patient without diabetes who received insulin experienced mild or severe hypoglycemia.
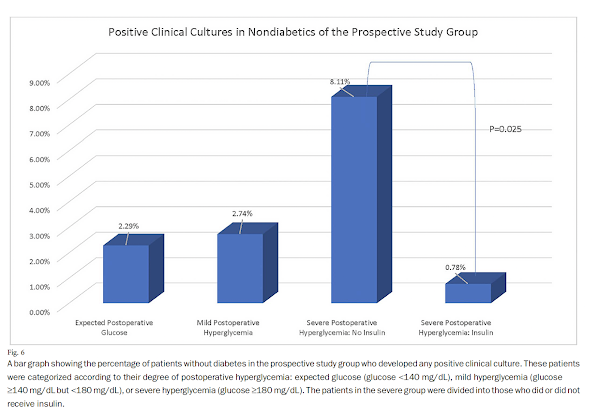
Insulin treatment reduced the frequency of positive cultures from any site (p = 0.025) and a composite of positive cultures and readmissions (p = 0.006) in comparison with no insulin treatment.
They concluded that postoperative hyperglycemia is frequent in patients without diabetes after orthopaedic surgery, but an enhanced glucose management program can lower mean postoperative glucose levels. The treatment of hyperglycemia in patients without diabetes reduced short-term complications and was associated with minimal side effects.
Comment: The results of this study indicate the importance of measuring the preoperative fasting blood glucose. Patients with elevated preoperative fasting glucose, perioperative steroid use, general anesthesia, long procedure duration, and transfusions should receive consideration for close postoperative monitoring and treatment of hyperglycemia.
Follow on twitter: https://twitter.com/shoulderarth
Follow on facebook: https://www.facebook.com/frederick.matsen
Follow on LinkedIn: https://www.linkedin.com/in/rick-matsen-88b1a8133/
How you can support research in shoulder surgery Click on this link.
Here are some videos that are of shoulder interest