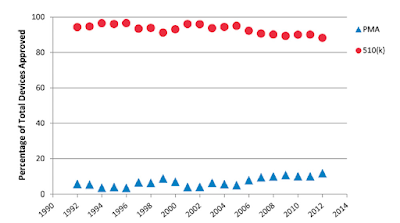
510(k) clearance does not require testing of safety and efficacy in clinical trials (see Shoulder Arthroplasty Device Clearance: An Ancestral Network Analysis). Instead 510(k) clearance requires evidence of “substantial equivalence” to a predicate device, i.e. one that has previously been cleared by the FDA.
Companies use the 510(k) premarket notification pathway for expedient approval of arthroplasty. With the passage of the 21st Century Cures Act, a piece of legislation reducing the rigor and amount of clinical testing required before device clearance, the 510(k) pathway has became further streamlined.
The number of shoulder implants approved by the 510(k) process is rising exponentially (see Is there evidence that the outcomes of primary anatomic and reverse shoulder arthroplasty are getting better?)

No fewer than twenty-nine new shoulder devices having received 510(k) premarket FDA approval over the 12-month period from 2020 – 2021.
Unfortunately, devices cleared through the 510(k) process are 11.5 times more likely to be recalled than devices approved via the more stringent Premarket Approval (PMA) process (see Analysis of FDA-Approved Orthopaedic Devices and Their Recalls). PMA approval is based on a determination by FDA that there is sufficient valid scientific evidence based on formal clinical trials to assure that the device is safe and effective.
Recalls of devices cleared by the 510(k) process typically occur years after the introduction of the device to clinical practice and after hundreds or thousands have been implanted into patients. Consider, for example, the ASR Acetabular & Resurfacing System which was recalled 6 years after being introduced into the market, after 90,000 had been implanted, and after 30% had been revised (see Out of joint: The story of the ASR).
Similarly, for three elbow implants, the lag between introduction to the market and recall ranged from 4 to 16 years, even though there were earlier reports of failure in the FDA's Manufacturer and User Facility Device Experience (MAUDE) data base (see Timely recognition of total elbow and radial head arthroplasty adverse events: an analysis of reports to the US Food and Drug Administration and Analysis of 4063 complications of shoulder arthroplasty reported to the US Food and Drug Administration from 2012 to 2016)

The authors of Shoulder Arthroplasty Device Clearance: An Ancestral Network Analysis sought to evaluate the FDA clearance of shoulder arthroplasty components by examining the interconnected ancestral network of shoulder arthroplasty devices and determining equivalency ties to devices that were subsequently recalled by the FDA because of design-related issues of relevance to patient safety.
They reviewed the FDA 510(k) database to identify all legally marketed shoulder arthroplasty devices from 5/28/1976 to 7/1/2021. Direct predicate information obtained via clearance summary documents associated with each device was used to generate an ancestral genealogy network for all shoulder arthroplasty devices cleared between 7/1/2020 and 7/1/2021. FDA design recalls were analyzed, and the number of descendent devices was calculated for each recalled device.
Their evaluation of all 476 shoulder devices cleared since 1976 identified between 0 and 313 descendent devices for each.
Of the 476 FDA cleared shoulder arthroplasty devices, 130 (27.3%) were linked to at least one predicate device that was subsequently recalled for issues with device design. Furthermore among 29 of the most-recently cleared devices (7/1/2020 – 7/1/2021), 16 (55.2%) were linked to predicate devices that have subsequently been withdrawn from the market due to design related failures. During this time interval no devices were cleared by the more vigorous PMA pathway.
80 of the devices (16.8%) cleared by the 510(k) pathway have since been recalled, of which 10 recalls were directly related to implant design issues (5 shoulder implant systems, 4 humeral components, and 1 glenoid component.) Of the ten devices recalled for device design, the most influential by number of ancestral descendants was K052906 (Zimmer Trabecular Metal Reverse Shoulder System) that had served as an ancestral predicate for 110 descendent devices.
One of the devices, (K080642 - Biomet Comprehensive Reverse Shoulder) was cleared by the FDA in 2008 and recalled in August 2017 due to higher than anticipated rates of implant breakage. K080642 - Biomet Comprehensive Reverse Shoulder served as an ancestral predicate for 67 descendant devices as shown in the figure below which indicates all descendants (Key: Red = recalled, yellow = direct predicate was recalled, green = not recalled and does not have a direct predicate that was recalled).

In 2011, the Institute of Medicine held a workshop that recommended replacement of the 510(k)-clearance process and implement in its place a regulatory premarket and postmarket regulatory framework to provide “a reasonable assurance of safety and effectiveness throughout the device life cycle.” In Congress, legislation to amend the 510(k)- approval process – the Safety of Untested and New Devices Act of 2012 – was introduced but failed to receive a vote on the floor.
Questions regarding the effectiveness of the FDA clearance process for orthopaedic devices have surfaced in the lay press. In 2018 the New York Times published, Can Your Hip Replacement Kill You?
This article tells the story of how orthopaedic implants can get 'cleared' by the FDA for use in patients without rigorous studies of their safety or effectiveness. The author states that the public "assumes that the Food and Drug Administration requires rigorous testing of medical devices before they are approved, the same as the lengthy approval process it requires for new drugs. In fact, most high-risk devices on the market, including implants, have undergone no clinical testing at all."
Orthopaedic surgeons should read the related book from cover to cover:
Comment: As orthopaedic surgeons, we are frequently presented with new implants that have just arrived on the marketplace, most of which have been 'cleared' by the 510(k) process because they are stated to be 'substantially equivalent' to a previously marketed device. However, these new devices cannot be 'completely equivalent' to implants that are currently available (otherwise they could not be patented and successfully marketed). The gap between 'substantial' and 'complete' equivalency allows for unexpected results and unanticipated complications as well as new technical challenges and learning curves in using the new system. Thus, when considering a new device, we need to ask "in what ways is the new thing different than what is currently in common use?" and "what are the potential adverse outcomes that might result from these differences" (see Assessing the Value to the Patient of New Technologies in Anatomic Total Shoulder Arthroplasty).
Finally, it seems that we should push for a better definition of "substantial equivalency'. Different bearing surfaces, different articular surface shapes, different materials, different fixation systems, and different degrees of modularity do not seem to be 'substantially equivalent'. Patient safety would seem to be served by a closer examination of systems that are not, in fact, substantially equivalent.
Comment: As orthopaedic surgeons, we are frequently presented with new implants that have just arrived on the marketplace, most of which have been 'cleared' by the 510(k) process because they are stated to be 'substantially equivalent' to a previously marketed device. However, these new devices cannot be 'completely equivalent' to implants that are currently available (otherwise they could not be patented and successfully marketed). The gap between 'substantial' and 'complete' equivalency allows for unexpected results and unanticipated complications as well as new technical challenges and learning curves in using the new system. Thus, when considering a new device, we need to ask "in what ways is the new thing different than what is currently in common use?" and "what are the potential adverse outcomes that might result from these differences" (see Assessing the Value to the Patient of New Technologies in Anatomic Total Shoulder Arthroplasty).
Finally, it seems that we should push for a better definition of "substantial equivalency'. Different bearing surfaces, different articular surface shapes, different materials, different fixation systems, and different degrees of modularity do not seem to be 'substantially equivalent'. Patient safety would seem to be served by a closer examination of systems that are not, in fact, substantially equivalent.
See this related post on device failure and FDA clearance.
You can support cutting edge shoulder research that is leading to better care for patients with shoulder problems, click on this link.
To add this blog to your reading list in Google Chrome, click on the reading list icon
Follow on twitter: https://twitter.com/shoulderarth
Follow on facebook: click on this link
Follow on facebook: https://www.facebook.com/frederick.matsen
Follow on LinkedIn: https://www.linkedin.com/in/rick-matsen-88b1a8133/
Here are some videos that are of shoulder interestShoulder arthritis - what you need to know (see this link).How to x-ray the shoulder (see this link).The ream and run procedure (see this link).The total shoulder arthroplasty (see this link).The cuff tear arthropathy arthroplasty (see this link).The reverse total shoulder arthroplasty (see this link).The smooth and move procedure for irreparable rotator cuff tears (see this link).Shoulder rehabilitation exercises (see this link).
Follow on twitter: https://twitter.com/shoulderarth
Follow on facebook: click on this link
Follow on facebook: https://www.facebook.com/frederick.matsen
Follow on LinkedIn: https://www.linkedin.com/in/rick-matsen-88b1a8133/
Here are some videos that are of shoulder interest
Shoulder arthritis - what you need to know (see this link).
How to x-ray the shoulder (see this link).
The ream and run procedure (see this link).
The total shoulder arthroplasty (see this link).
The cuff tear arthropathy arthroplasty (see this link).
The reverse total shoulder arthroplasty (see this link).
The smooth and move procedure for irreparable rotator cuff tears (see this link).
Shoulder rehabilitation exercises (see this link).