These authors assessed clinical and radiographic outcomes at midterm follow-up of anatomic total shoulder arthroplasty using a Zimmer Biomet trabecular metal glenoid component placed with full backside support using an inset technique. The glenoid components were used in a press-fit manner, which is "off label" in the U.S..
Of 39 patients who having 41 total shoulder arthroplasty procedures with the Zimmer glenoid component, 35 patients (37 shoulders) were available for a minimum 2 year (average 7.2 years, range, 2-11) clinical follow-up.
At final follow-up, the average shoulder elevation was 153 ± 22° and external rotation was 53 ± 12°. Average ASES scores were 86.8 ± 19.0, and VAS scores were 1.3 ± 2.4. Nine shoulders (27%) had metallic debris. Metal debris was not associated with inferior clinical outcomes.
Comment: This study of minimum 2 year results shows that good outcomes can be obtained with a trabecular metal glenoid component in type A glenoid pathology; this type of pathoanatomy is the most straightforward of all the glenoid types. The study does not provide data on the use of this implant for the more problematic glenoid types: B1, B2, B3, C, and D.
Even for the type A glenoids, the study does not provide evidence that the trabecular metal component is superior to the commonly used all-polyeythene component. For example, this study found a 39% rate of radiolucency, which is not lower than that reported for all-polyethylene glenoids (see this link).
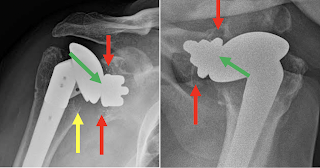
In one of the examples shown in the paper, there is a small amount of metal debris, but apparently secure component fixation with a substantial radiographic joint space (green arrows), centering of the humeral head in the glenoid and no evidence of osteolysis or stress shielding.
Follow on twitter: https://twitter.com/shoulderarth
Follow on facebook: https://www.facebook.com/frederick.matsen
Follow on LinkedIn: https://www.linkedin.com/in/rick-matsen-88b1a8133/
How you can support research in shoulder surgery Click on this link.
Here are some videos that are of shoulder interest