The FDA cleared the first reverse total shoulder arthroplasty (rTSA) for use in the United States in late 2003 (Delta Shoulder K021478; DePuy Inc., Raynham, MA, USA). The FDA approved on-label Indications for Use for rTSA at that time were limited to cuff tear arthropathy and revision surgery: “Grossly rotator cuff deficient joint with severe arthropathy or a previous failed joint replacement with a grossly rotator cuff deficient joint. The patient’s joint must be anatomically and structurally suited to receive the selected implant(s), and a functional deltoid muscle is necessary to use the device" (see shoulder prosthesis, reverse configuration and FDA clearance).
After the clearance for rTSA use for rotator cuff tear arthropathy, surgeons began to use rTSA for off-label indications (i.e. those other than the original approved indications), such as osteoarthritis without rotator cuff tear, massive cuff tear without osteoarthritis, proximal humerus fractures, tumor, inflammatory arthritis, and chronic glenohumeral joint dislocation. Since 2006, some devices were subsequently cleared by the FDA for use in proximal humeral fractures and other indications; on the other hand, some reverse shoulder devices remain cleared only for the initial indications.
The authors of Off Label use of Reverse Total Shoulder Arthroplasty: The American Academy of Orthopedic Surgeons Shoulder and Elbow Registry evaluated the trends for the rTSA in the United States with respect to those uses that were consistent with the original FDA approval and those which were "off label". They analyzed 3850 rTSA procedures reported to the AAOS shoulder and elbow registry from Jan 2015-Mar 2021.
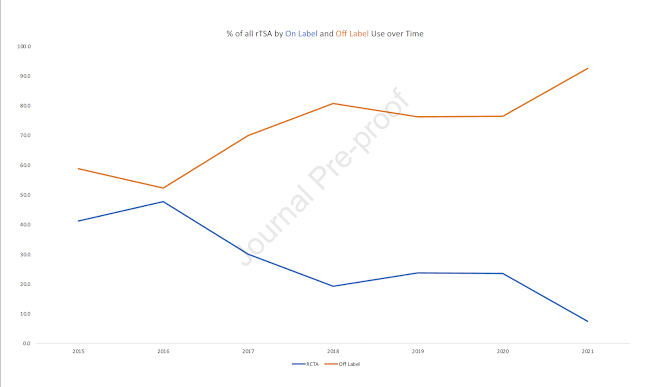
They found that only 24.4% of rTSA surgeries were performed for original on-label use (rotator cuff tear arthropathy). Off-label use of rTSA was seen in 75.6% of cases. Furthermore, they found that off-label use is increasing over time while on-label use is decreasing.
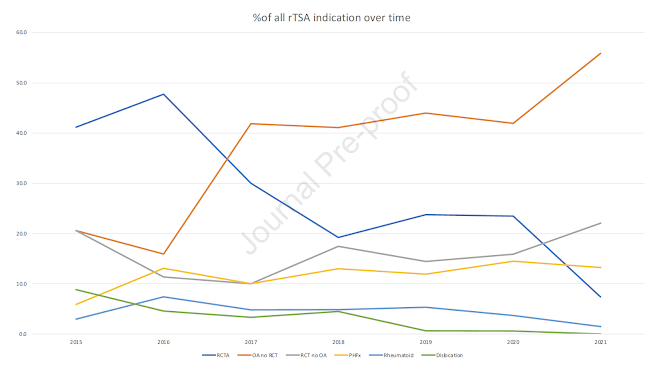
When reviewing those rTSA done off-label, the majority (41.4%) were done for osteoarthritis without cuff tear. Other off-label rTSA use included 15.1% for cuff tear without arthritis, 13% potentially off-label for proximal humeral fractures, 4.6% for inflammatory arthropathy and 1.6% for glenohumeral dislocation.
The authors point out that "some implant manufacturers have expanded indications for rTSA without providing clinical data to support changing FDA approved Indications for Use. They presented the table below showing the approved and unapproved indications for different implants.
"Only 10-15% of the 510(k) premarket notification applications are supported by clinical data. The incremental expansion of Indications for Use without supportive data, a practice known as predicate creep, is occurring with rTSA. Performing rTSA for off-label indications may create liability risk for surgeons and implant manufacturers.".
The FDA does allow for some off-label use of devices stating: “Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics, and devices according to their best knowledge and judgement. If physicians use a product for an indication not in the approved labeling, they have the responsibility to be well informed about the product, to base its use on firm scientific rationale and on sound medical evidence, and to maintain records of the product's use and effects”
Despite the leeway the FDA provides in permitting surgeons to use devices off-label under practice of medicine, surgeons in the United States may still be at risk for litigation when using rTSA for an off-label indication, particularly if they have a financial relationship with the company or other conflict of interest, or the informed consent does not document that the off label use of the device was discussed with the patient.
Comment: These authors conclude that the current Indications for Use of rTSA are confusing and not uniform among systems. They recommend that device manufacturers pursue labelling changes by the FDA supported by the clinical data needed to formally expand these indications.
You can support cutting edge shoulder research that is leading to better care for patients with shoulder problems, click on this link.
Follow on twitter: https://twitter.com/shoulderarth
Follow on facebook: click on this link
Follow on facebook: https://www.facebook.com/frederick.matsen
Follow on LinkedIn: https://www.linkedin.com/in/rick-matsen-88b1a8133/
Here are some videos that are of shoulder interest
Shoulder arthritis - what you need to know (see this link).
How to x-ray the shoulder (see this link).
The ream and run procedure (see this link).
The total shoulder arthroplasty (see this link).
The cuff tear arthropathy arthroplasty (see this link).
The reverse total shoulder arthroplasty (see this link).
The smooth and move procedure for irreparable rotator cuff tears (see this link).
Shoulder rehabilitation exercises (see this link).